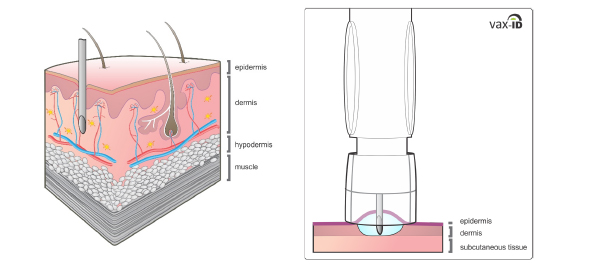
How does VAX-ID work?
Interested in our solutions?
Contact our commercial team!
VAX-ID®: An innovative solution for reliable intradermal drug delivery
VAX-ID® is an award-winning patented intradermal drug delivery device with a high ease of use. It is suited for standardized, accurate and reliable intradermal injection, i.e. in the dermal layer of the skin. VAX-ID is applied perpendicular to the skin by a healthcare professional.
VAX-ID contains a short and thin, almost invisible needle. Therefore, it is considered an advantage for people with needle phobia and make people feel more at ease during the injection feeling no pain.
Controlled penetration depth
Accuracy of penetration depth is important as the dermal layer of the skin limited in thickness. Importantly, the penetration depth of the needle was predefined based on skin thickness evaluations executed in adults, adolescents as well as children (Van Mulder et al. 2017 and 2020)
Why use VAX-ID?
Developed by a multidisciplinary team, VAX-ID® allows for
- Accurate injection at a predefined penetration depth
- Dose sparing
- Activation protection and needle-stick injury prevention
- Low in pain and no needle phobia
- Easy to use, potentially leading to self-administration
- User independent
VAX-ID device platform
VAX-ID® can be preconfigured with a 32G, 30G, or 27G needle with a penetration depth of 0.85, 1.00mm and 1.2mm, respectively. The devices allow for the delivery of small volumes (0.05cc – 0.2cc per injection).
Devices are currently available for investigational use only (IUO) and research use only (RUO). CE marking and FDA approval are expected in the upcoming months.